10% DISCOUNT ON ANNUAL MEMBERSHIP FEE IF YOU REFER A NEW MEMBER
Contact info@emwa.org
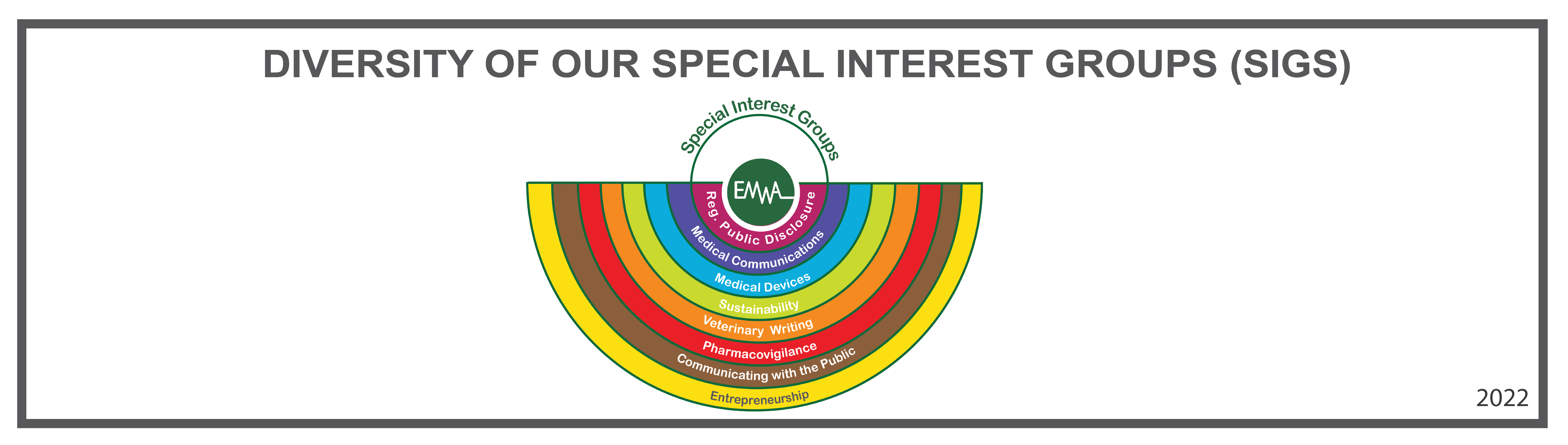
EMWA is the European Medical Writers Association, a network of professionals representing, supporting, and training medical communicators.
It is a not-for-profit organisation run for its members by its members.
This website offers access to EMWA's journal Medical Writing, our Freelancer and Company listing, and our job vacancies (see below) for freelance and in-house medical writers and editors.
If you want more information about signing up to the freelancer or company list or advertising your job vacancies on this website, please contact info@emwa.org.